Double Blind Study Definition
Start studying blind and double blind study. This approach is frequently used in the research.
Double blind trials are thought to produce objective results since the expectations of the researcher and the participant about the experimental treatment such as a drug do not affect the outcome.

Double blind study definition. Term used to described a study in which both the investigator or the participant are blind to unaware of the nature of the treatment the participant is receiving. What does double blind experiment mean. Double blind definition is of relating to or being an experimental procedure in which neither the subjects nor the experimenters know which subjects are in the test and control groups during the actual course of the experiments.
A technique for eliminating subjective bias from the test results. A medical study in which both the subjects participating and the researchers are uaware of when the experimental medication or procedure has been given. Experimental procedure the specific techniques used in conducting a particular experiment.
A testing procedure designed to eliminate biased results in which the identity of those receiving a test treatment is concealed from both administrators and subjects until after the study is completed. Supplies are prepared for treatment a active and indistinguishable placebo and for treatment b active and indistinguishable placebo. Double blind studies are an important part of conducting research.
Meaning of double blind study. An experimental procedure where the nature of the experiment is not known. Compare single blind triple blind.
Double dummy technique double dummy is a technique for retaining the blind when administering supplies in a clinical trial when the two treatments cannot be made identical. This procedure is utilized to prevent bias in research results. A study of the effects of a specific agent in which neither the administrator nor the recipient at the time of administration knows whether the active or an inert substance is given.
Of or relating to an experiment or clinical trial in which neither the subjects nor the researchers know which subjects are receiving the active medication treatment etc and which are not. Continue scrolling or click here for related article. Double blind study an experimental procedure in which neither the subjects of the experiment nor the persons administering the experiment know the critical aspects of the experiment.
Double blind study listen duh bul blind stuh dee a type of clinical trial in which neither the participants nor the researcher knows which treatment or intervention participants are receiving until the clinical trial is over. Double blinded studies are often used when initial studies shows particular promise. These terms describe experiments in which respectively one two or three parties are blinded to some information.
Learn how double blind studies contribute to the validity of research by reducing the biases of research participants and the. Learn vocabulary terms and more with flashcards games and other study tools. It is called this because two parties are kept in the dark about the experiment.
An experiment of this type is said to be double blind. Psychology definition of double blind. Adjective of relating to or being an experimental procedure in which the experimenters but not the subjects know the makeup of the test and control groups during the actual course of the experiments compare double blind open label.
In medical research the terms single blind double blind and triple blind are commonly used to describe blinding. Double blind experiment an experimental procedure in which neither the subjects of the experiment nor the persons administering the. In some circumstances particularly families and relationships this might be emotionally distressing.
What does double blind study mean. Information and translations of double blind experiment in the most comprehensive dictionary definitions resource on the web. Double blind experiment double blind procedure type of.
Double blind experiment double blind procedure. If well designed they provide the strongest possible evidence of causation 2 3 to understand this clearly it is necessary to elaborate upon the key words used in the above statement. When undertaking a clinical trial the two major models that one can use are the single blind and double blind trials.
Double blind studies are particularly useful for preventing bias due to demand characteristics or the placebo effect. Double blind placebo controlled clinical trial thus a double blind placebo controlled clinical trial is a medical study involving human participants in which neither side knows whos getting what treatment and placebo are given to a control group. Pharmaceutical drug trials offer a good example of when double blind experiments are especially useful.
A double blind study or experiment compares two groups of people one of which is being. A double blind procedure is used to guard against both experimenter bias and placebo effects. When this is part of the experimental design it is called a double blind experiment.
What is the difference between single blind and double blind clinical trials. Selecting the right trial is important since it can affect the outcome of the trial or introduce errors. The double blind experiment takes this precaution against bias one step further by ensuring that the researcher does not know in which group a patient falls.
A double blind study is a study in which both the person implementing the experiment and the participants are not aware of which individual is receiving the experimental treatment. This makes results of the study less likely to be biased. Randomized double blind placebo control rdbpc studies are considered the gold standard of epidemiologic studies.
Meaning pronunciation translations and examples. Both the subject and the person administering the treatment do not know whether the subject in the experimental or control group. Information and translations of double blind study in the most comprehensive dictionary definitions resource on the web.
1 n an experimental procedure in which neither the subjects of the experiment nor the persons administering the experiment know the critical aspects of the experiment synonyms. Whilst the vast majority of researchers are professionals there is always a chance that the researcher might subconsciously tip off a patient about the pill they were receiving. Meaning of double blind experiment.
Double masked study a type of clinical trial in which neither the participants nor the research team know which treatment a specific participant is receiving. Double blind experiment synonyms double blind experiment pronunciation double blind experiment translation english dictionary definition of double blind experiment. A double bind is a dilemma in communication in which an individual or group receives two or more conflicting messages with one negating the other.
A double blind study is one in which neither the participants nor the experimenters know who is receiving a particular treatment. The purpose of a double blind experiment is to ensure that the results are not biased.
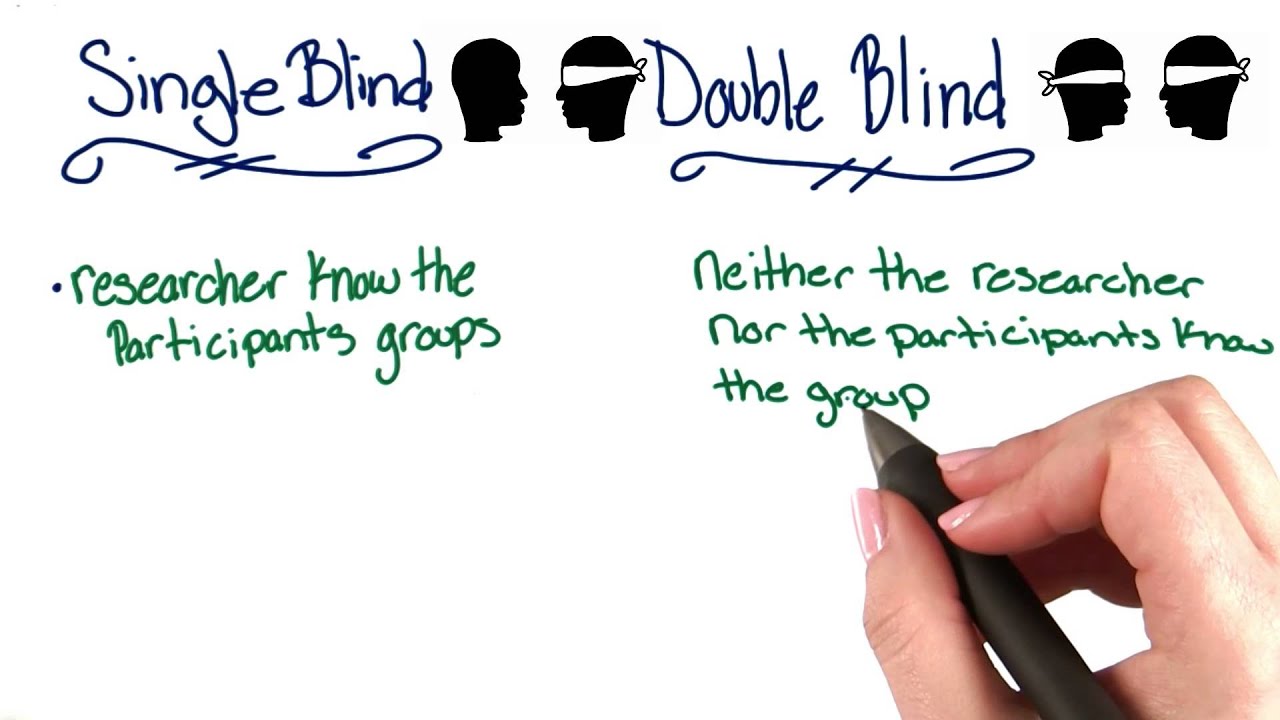 Double Blind Studies Intro To Psychology
Double Blind Studies Intro To Psychology
 Blind Study Definition Explanation Video With Lesson
Blind Study Definition Explanation Video With Lesson
 Double Blind Study Definition Explanation Video With
Double Blind Study Definition Explanation Video With
 Double Blind Studies Intro To Psychology Youtube
Double Blind Studies Intro To Psychology Youtube
 Double Blinded Study The Investigators And Participants In
Double Blinded Study The Investigators And Participants In
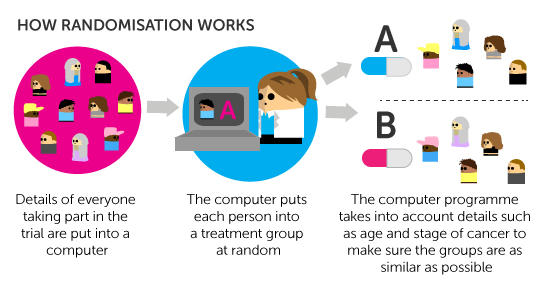 Randomised Trials Cancer Research Uk
Randomised Trials Cancer Research Uk
 Quiz Worksheet Double Blind Studies Study Com
Quiz Worksheet Double Blind Studies Study Com
 On Biostatistics And Clinical Trials Double Dummy Technique
On Biostatistics And Clinical Trials Double Dummy Technique

 Understanding Research Study Designs Health Sciences Libraries
Understanding Research Study Designs Health Sciences Libraries
 Understanding Research Study Designs Health Sciences Libraries
Understanding Research Study Designs Health Sciences Libraries
 Comparison Of Double Blind Randomized Placebo Controlled
Comparison Of Double Blind Randomized Placebo Controlled
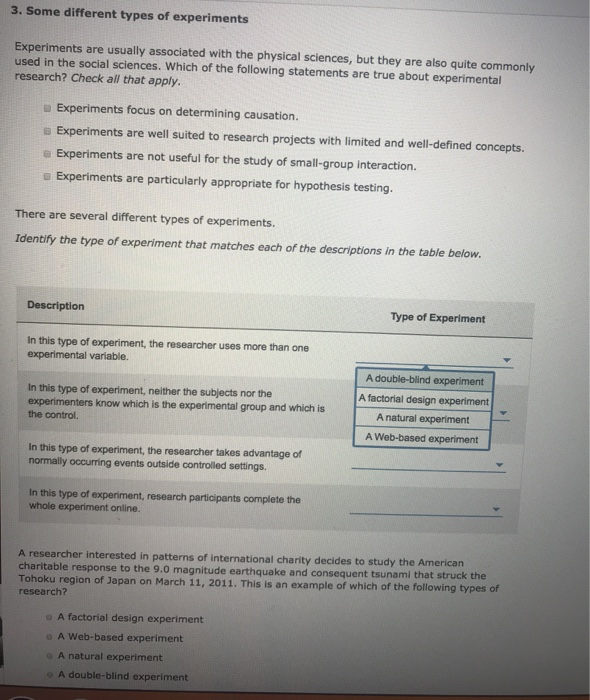 3 Some Different Types Of Experiments Experiments
3 Some Different Types Of Experiments Experiments
 Spravato Esketamine Ciii Efficacy Clinical Trial Design
Spravato Esketamine Ciii Efficacy Clinical Trial Design
 Figure 2 From Md1003 High Dose Biotin For The Treatment Of
Figure 2 From Md1003 High Dose Biotin For The Treatment Of
 Tresiba Switch 2 Trial Tresiba Insulin Degludec
Tresiba Switch 2 Trial Tresiba Insulin Degludec
 Randomized Controlled Trial Wikipedia
Randomized Controlled Trial Wikipedia
 Randomized Controlled Trial Wikipedia
Randomized Controlled Trial Wikipedia
 Pdf Multicentre Double Blind Study For Evaluation Of
Pdf Multicentre Double Blind Study For Evaluation Of
Regenerate A Phase 3 Double Blind Randomized Placebo
 Safety And Efficacy Of Edaravone In Well Defined Patients
Safety And Efficacy Of Edaravone In Well Defined Patients
 Descovy Study Design Stably Suppressed Adults
Descovy Study Design Stably Suppressed Adults
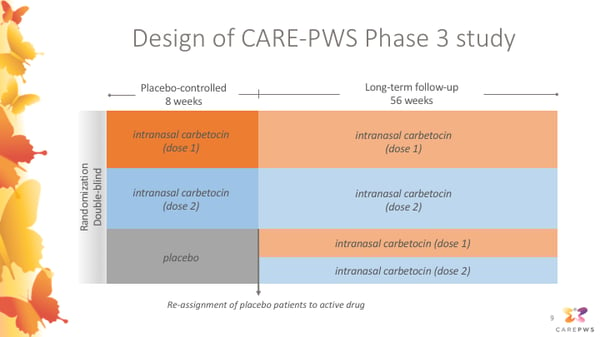 Pws Clinical Trial Webinar Carbetocin
Pws Clinical Trial Webinar Carbetocin
Study Design Rubraca Rucaparib Tablets
 Spravato Esketamine Ciii Efficacy Clinical Trial Design
Spravato Esketamine Ciii Efficacy Clinical Trial Design
 Phase Iii Pivotal Clinical Trial Plegridy Peginterferon
Phase Iii Pivotal Clinical Trial Plegridy Peginterferon
 Figure 3 From Exploratory Double Blind Parallel Group
Figure 3 From Exploratory Double Blind Parallel Group
 Chapter 6 Study Design Ppt Video Online Download
Chapter 6 Study Design Ppt Video Online Download
Blind And Double Blind Study Research Paper Example August
H8h Cd Lahk A Study Of Three Doses Of Lasmiditan 50 Mg 100
 Understanding Research Study Designs Health Sciences Libraries
Understanding Research Study Designs Health Sciences Libraries
 What Is A Single Blind Study Explore Psychology
What Is A Single Blind Study Explore Psychology
 Evidence Of Experimental Bias In The Life Sciences Why We
Evidence Of Experimental Bias In The Life Sciences Why We
:max_bytes(150000):strip_icc()/what-is-a-cross-sectional-study-2794978-v1-c70f858baccc46c698b6ca6ea8be718a.png) The Definition And Use Of A Cross Sectional Study
The Definition And Use Of A Cross Sectional Study
 Experiments Vs Observational Studies Definition
Experiments Vs Observational Studies Definition
 Solved A Study Used Nicotine Gum To Help People Quit Smok
Solved A Study Used Nicotine Gum To Help People Quit Smok
 Learn More About Clinical Trials Clinical Trials
Learn More About Clinical Trials Clinical Trials
 Treatment Naive Patient Clinical Trial Results Symtuza
Treatment Naive Patient Clinical Trial Results Symtuza
 Duet A Phase 2 Study Evaluating The Efficacy And Safety Of
Duet A Phase 2 Study Evaluating The Efficacy And Safety Of
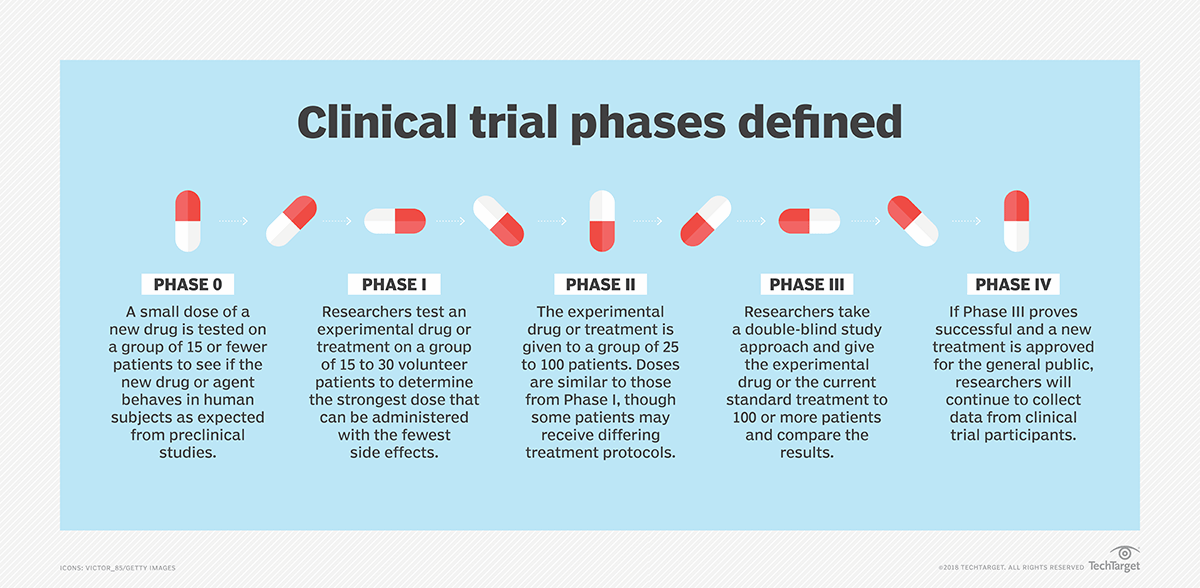 What Is Clinical Trial Definition From Whatis Com
What Is Clinical Trial Definition From Whatis Com
 How To Process Data From Clinical Trials And Their Open
How To Process Data From Clinical Trials And Their Open
 Design Of Major Randomized Trials Part 3 Of A 4 Part Series
Design Of Major Randomized Trials Part 3 Of A 4 Part Series
 Phase 3 Randomized Placebo Controlled Double Blind Study
Phase 3 Randomized Placebo Controlled Double Blind Study

 Summary Of Adhd Pediatric Withdrawal Studies Response And
Summary Of Adhd Pediatric Withdrawal Studies Response And
 Background Research At University Of Denver Studyblue
Background Research At University Of Denver Studyblue
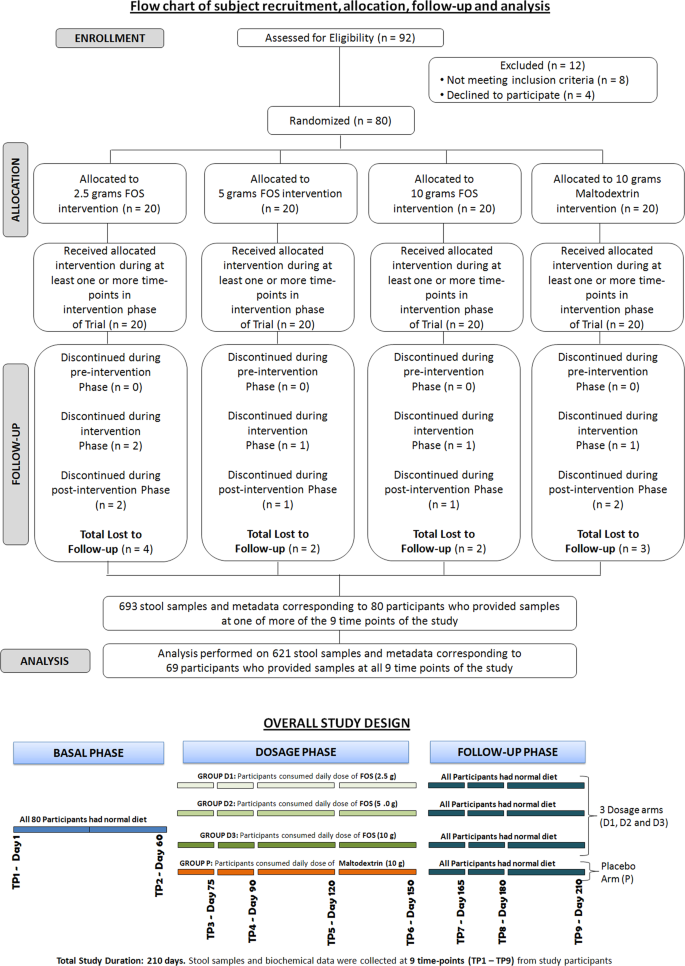 A Prospective Randomized Double Blind Placebo Controlled
A Prospective Randomized Double Blind Placebo Controlled
 Double Blind Faq Ieee Secure Development Conference
Double Blind Faq Ieee Secure Development Conference
/cdn.vox-cdn.com/uploads/chorus_asset/file/8807955/PLACEBO_LEAD.jpg) The Weird Power Of The Placebo Effect Explained Vox
The Weird Power Of The Placebo Effect Explained Vox
 Randomised Double Blind Placebo Controlled Study Of
Randomised Double Blind Placebo Controlled Study Of
 Antibiotic Treatment Of Acute Otorrhea Through Tympanostomy
Antibiotic Treatment Of Acute Otorrhea Through Tympanostomy
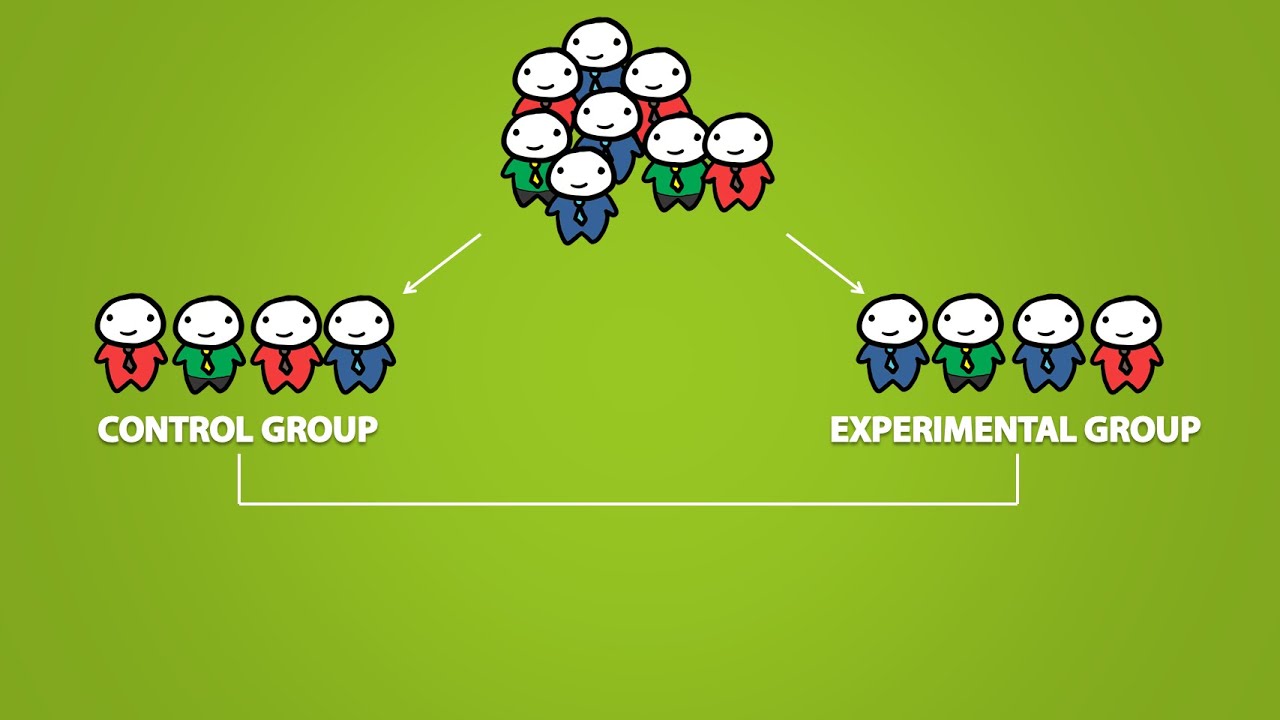 Placebo Effect Control Groups And The Double Blind Experiment 3 2
Placebo Effect Control Groups And The Double Blind Experiment 3 2
 A Prospective Randomized Double Blind Placebo Controlled
A Prospective Randomized Double Blind Placebo Controlled
Placebo Effect Of Medication Cost In Parkinson Disease
 Figure 1 From A Randomized Double Blind Placebo Controlled
Figure 1 From A Randomized Double Blind Placebo Controlled
 Crohn S Disease Cd Clinical Trial Study Design
Crohn S Disease Cd Clinical Trial Study Design
 Between Group Design Wikipedia
Between Group Design Wikipedia
 Crossover Study An Overview Sciencedirect Topics
Crossover Study An Overview Sciencedirect Topics
 Clinical Trials Information For Ovarian Cancer Treatment
Clinical Trials Information For Ovarian Cancer Treatment
Imerge Study Myelodysplastic Syndromes Clinical Trial

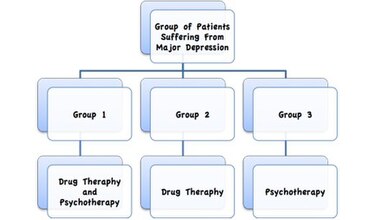
 Week 7 Quiz Answers Docx 1 Evaluation Of Evidence Should
Week 7 Quiz Answers Docx 1 Evaluation Of Evidence Should
 Lithium In The Acute Treatment Of Bipolar I Disorder A
Lithium In The Acute Treatment Of Bipolar I Disorder A
 A Multicenter Randomized Double Blind Placebo Controlled
A Multicenter Randomized Double Blind Placebo Controlled
 Correlational Studies In Psychology Examples Advantages
Correlational Studies In Psychology Examples Advantages
 Siponimod Versus Placebo In Secondary Progressive Multiple
Siponimod Versus Placebo In Secondary Progressive Multiple
Words That Describe How A Study Is Done Understanding Research
 Duet A Phase 2 Study Evaluating The Efficacy And Safety Of
Duet A Phase 2 Study Evaluating The Efficacy And Safety Of
Evidence Of Experimental Bias In The Life Sciences Why We
 On Biostatistics And Clinical Trials Double Dummy Technique
On Biostatistics And Clinical Trials Double Dummy Technique
 11 Tough Vocab Terms For Ap Psychology Research Methods
11 Tough Vocab Terms For Ap Psychology Research Methods
Looking At The Evidence Video The Wise Old Man Blog
 Tresiba Switch 2 Trial Tresiba Insulin Degludec
Tresiba Switch 2 Trial Tresiba Insulin Degludec
 Phase Iii Pivotal Clinical Trial Plegridy Peginterferon
Phase Iii Pivotal Clinical Trial Plegridy Peginterferon
Work Instruction Study Registration
 A Randomized Double Blind Study Of Phenytoin For The
A Randomized Double Blind Study Of Phenytoin For The
 Definition Of Double Blind Study Nci Dictionary Of Cancer
Definition Of Double Blind Study Nci Dictionary Of Cancer
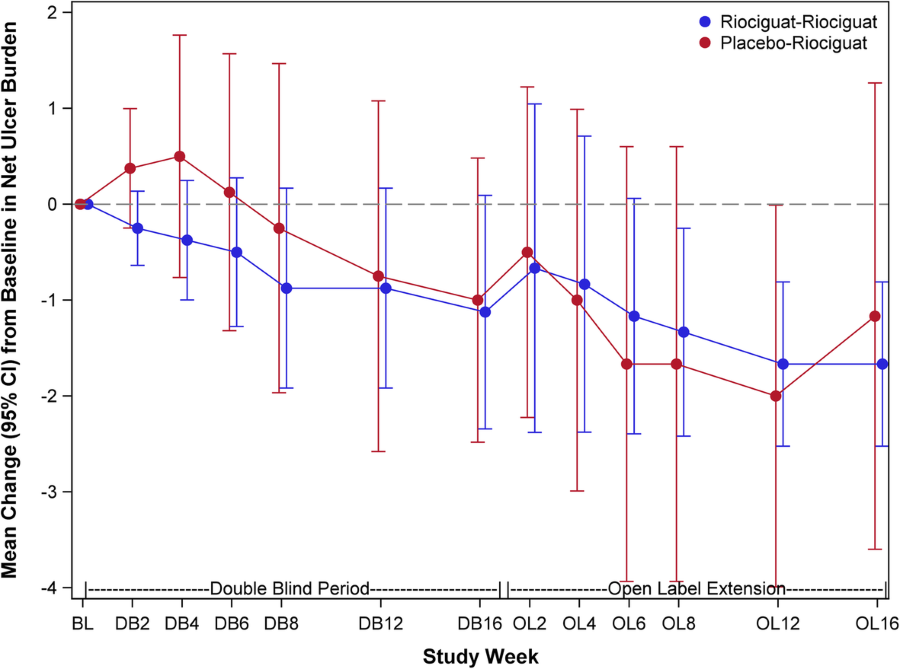
 Abstract 3663 Use Of Inhaled Nitric Oxide After Left
Abstract 3663 Use Of Inhaled Nitric Oxide After Left
 Corporate Presentation January 2013 Otc Qb Tnxp Tonix
Corporate Presentation January 2013 Otc Qb Tnxp Tonix
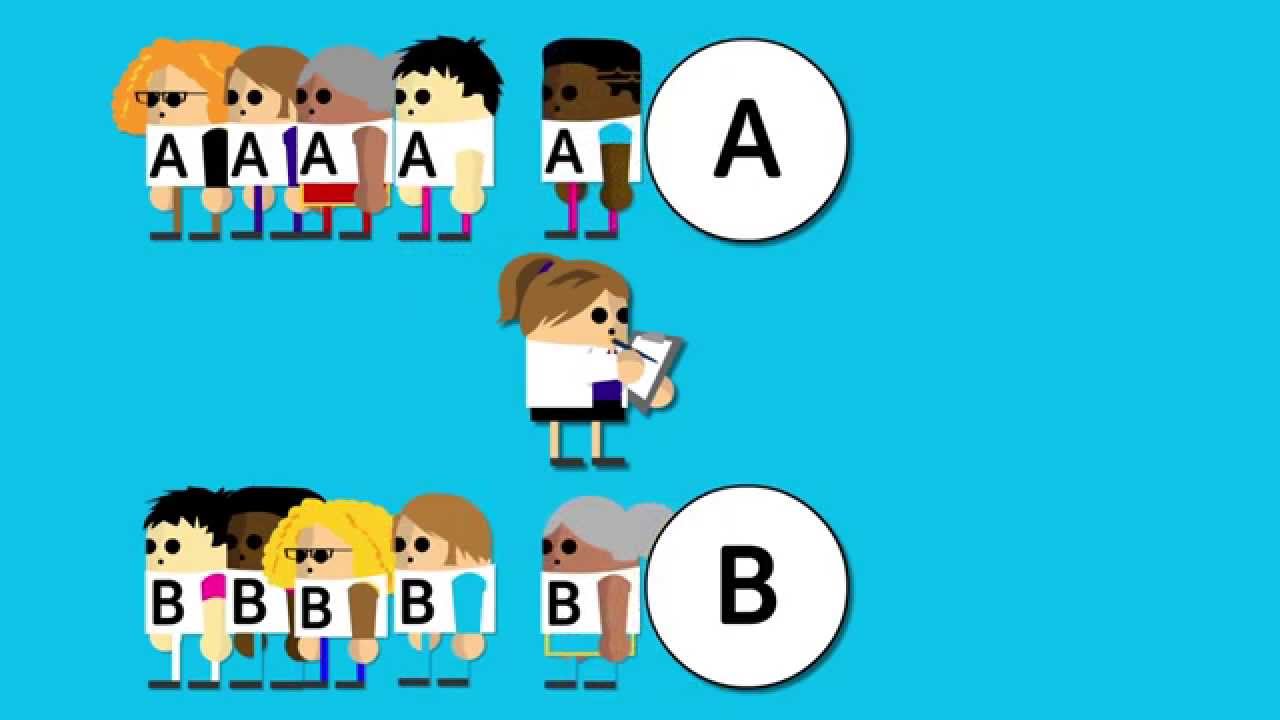 Randomised Trials Cancer Research Uk
Randomised Trials Cancer Research Uk
A Randomized Double Blind Placebo Controlled Trial Of
Definition Of Blind Study Double Blind Dkrs Group
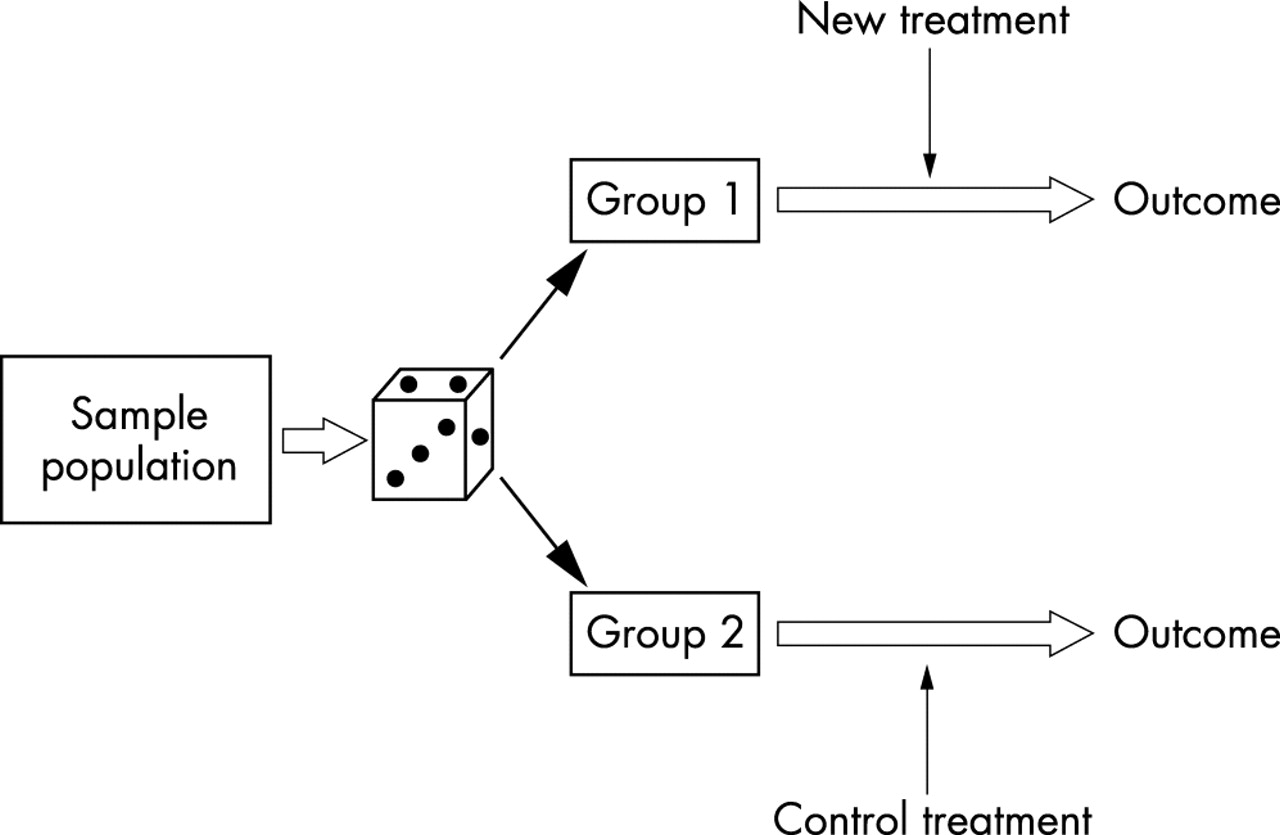 Designing A Research Project Randomised Controlled Trials
Designing A Research Project Randomised Controlled Trials
 Different Types Of Clinical Trials S E F O
Different Types Of Clinical Trials S E F O
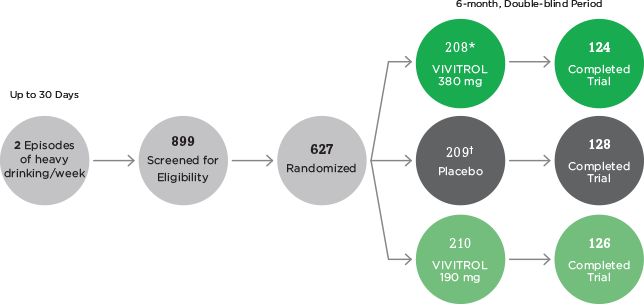 Alcohol Dependence Pivotal Study Data
Alcohol Dependence Pivotal Study Data
 Understanding Research Study Designs Health Sciences Libraries
Understanding Research Study Designs Health Sciences Libraries
 Bertrand Delsuc On Twitter Ablynx Ablx Results Of The
Bertrand Delsuc On Twitter Ablynx Ablx Results Of The
 A Randomized Double Blind Study Of Phenytoin For The
A Randomized Double Blind Study Of Phenytoin For The
 Solved To Minimize The Extent To Which Outcome Difference
Solved To Minimize The Extent To Which Outcome Difference
 Effects Of Microbiota Directed Foods In Gnotobiotic Animals
Effects Of Microbiota Directed Foods In Gnotobiotic Animals
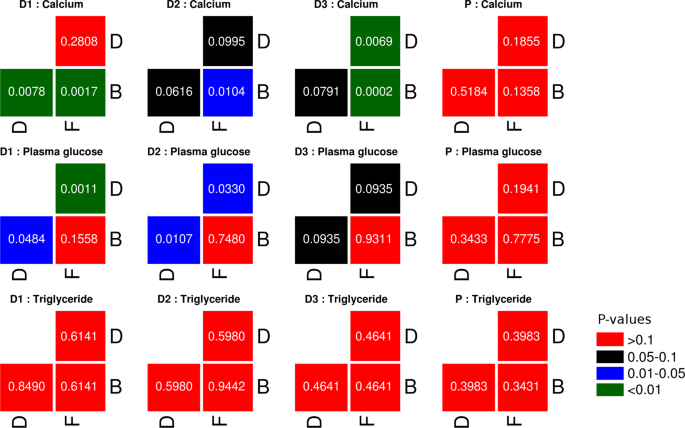
Biomedicine And Nursing 2015 1 2 Http Www Nbmedicine Org
Different Types Of Clinical Trials S E F O
:max_bytes(150000):strip_icc()/what-is-a-double-blind-study-2795103_color1-5bf4763f4cedfd002629e836.png)

:max_bytes(150000):strip_icc()/GettyImages-492759848-5acfa6f6fa6bcc00364cfcf7.jpg)



0 Response to "Double Blind Study Definition"
Post a Comment